August 15, 2014—Westminster, CO—Surefire Medical, Inc., a commercial stage medical device company developing innovative devices for minimally invasive, direct-to-tumor interventional procedures, today announced that Andor F. van den Hoven, MD of University Medical Center Utrecht, The Netherlands, has been selected as the first recipient of the Fellows Outstanding Case Study Award.
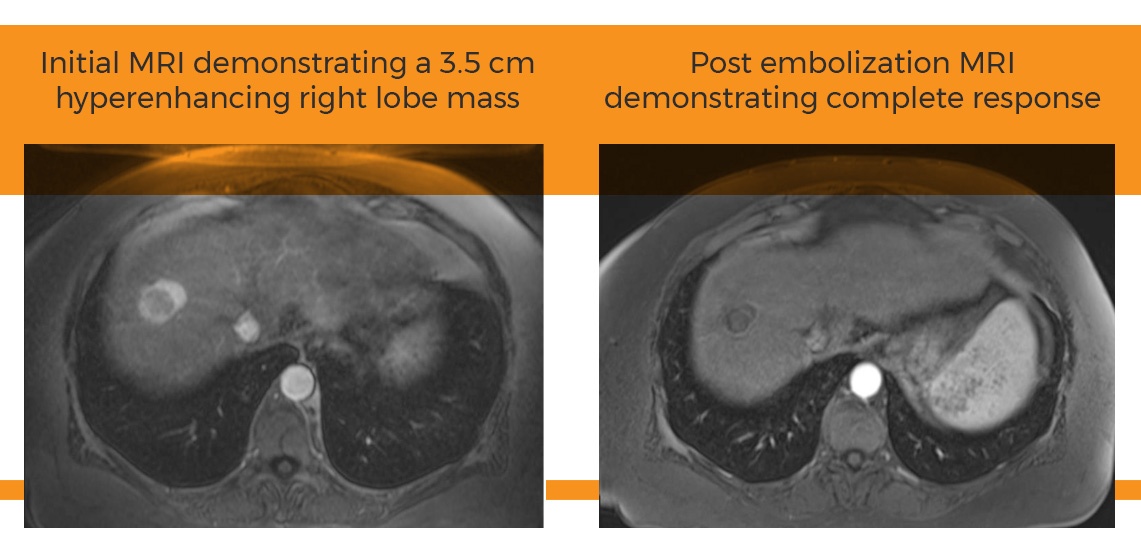
The case study submitted by Dr. van den Hoven, titled “Selective 90Y-Radioembolization in a Replaced Left Hepatic Artery,” was selected by Surefire’s Scientific Advisory Board based upon its clinical merit, success of use with the Surefire Infusion System, proper use and originality.
“We are pleased to recognize the work of Dr. van den Hoven with our first case study award and value this type of collaboration with fellows since they are the future of Interventional Radiology,” says Surefire President and CEO Jim Chomas.
Surefire Medical launched the Fellows Outstanding Case Study Program earlier this year to support new physicians joining the Interventional Radiology community and to highlight relevant clinical experiences with the advanced technology for treating some of the 80 percent of liver cancer patients with inoperable tumors. Submissions are accepted on an ongoing basis and quarterly awards presented to the selected recipient. Awarded fellows will be recognized at Surefire’s Annual “Life After Fellowship” meeting and present to their peers in Atlanta during the 2015 Annual Society of Interventional Radiology (SIR) Scientific Meeting.
To learn more about the program, please visit http://surefiremedical.com/fellows.
About Surefire Medical
Surefire Medical, Inc., based in Westminster, Colo., was founded in 2009 to develop innovative infusion systems for the interventional radiology and interventional oncology markets. Surefire’s infusion systems are designed to precisely deliver embolic agents through a unique microcatheter with an expandable tip that collapses in forward flow and dynamically expands to the vessel wall in reverse flow in order to maximize targeted delivery, minimize reflux and reduce damage to healthy tissue. The Surefire Infusion Systems have received regulatory approval in the U.S., Europe, Canada, Australia, New Zealand and Taiwan. www.surefiremedical.com