Preliminary Observations Presented at GEST Show Increased Tumor Penetration with the Surefire
May 1, 2014—San Francisco, CA—Surefire Medical, Inc. announced today that early observations from the first direct comparison of the Surefire Infusion System vs. a standard end hole catheter in vivo will be presented at the Global Embolization Symposium and Technologies (GEST) meeting in San Francisco May 1-4.
The University of Tennessee Medical Center prospective study uses a new dual step infusion technique to compare biodistribution after the Surefire Infusion System is used and after use of a conventional end hole catheter. Patients undergo two infusion procedures on the same day in order to eliminate sources of uncertainty other than the type of catheter used. Particle uptake and distribution are assessed with SPECT imaging after each procedure.
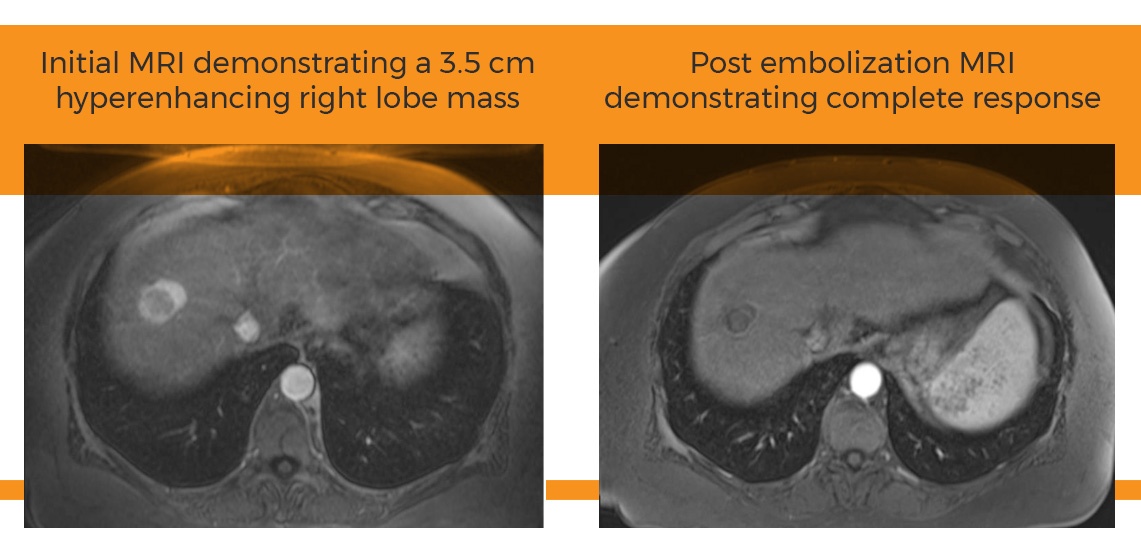
“Nuclear imaging is more quantifiable than other imaging techniques,” says study author Alexander Pasciak, Ph.D. “The observations are preliminary but in the first five patients, nuclear imaging shows greater penetration and decreased non-target embolization using the Surefire Infusion System.” The images can be viewed here.
Tumor penetration is also assessed on the day of radioembolization therapy. Bremsstrahlung SPECT and 90Y PET/CT scans show particle distribution with the Surefire system closely matched pretreatment planning intentions.
The latest clinical evidence builds on previous research showing that the Surefire’s unique, pliable expanding tip controls downstream hepatic arterial blood pressure changes that potentially increase tumor uptake of embolization agents.
“This trial is exciting because it directly compares infusion systems in the same patient, same disease and same day. Early data suggests the choice of infusion system plays a big role in dose delivered into a tumor,” says Surefire President and CEO Jim Chomas. “The possibility of potentially greater tumor penetration – with its implications for increasing efficiency and reducing costs of embolization procedures—continues to build the case for preferential use of the novel Surefire Infusion System”
The latest study comes on the heels of the recently presented COSY trial, which found significantly reduced procedure time, fluoroscopy time and contrast dose when using the Surefire system to embolize liver cancers.
About Surefire Medical
Surefire Medical, Inc., based in Westminster, Colo., was founded in 2009 to develop innovative infusion systems for the interventional radiology and interventional oncology markets. Surefire’s infusion systems are designed to precisely deliver embolic agents through a unique microcatheter with an expandable tip that collapses in forward flow and dynamically expands to the vessel wall in reverse flow in order to maximize targeted delivery, minimize reflux and reduce damage to healthy tissue. The Surefire Infusion Systems have received regulatory approval in the U.S., Europe, Canada, Australia, New Zealand and Taiwan. www.surefiremedical.com