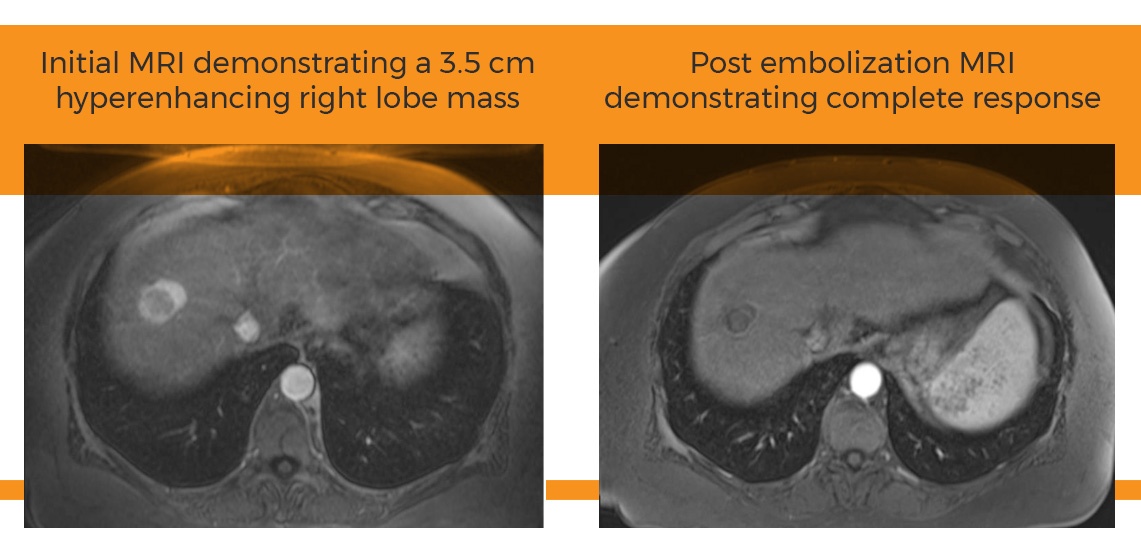
Study presented at SIR: Surefire results in statistically significant tumor uptake
March 3, 2015—Atlanta, GA—Surefire Medical, Inc. today announced the results of a prospective clinical trial comparing the Surefire Infusion System with an end hole microcatheter. In patients with either primary or metastatic liver cancer, using the Surefire resulted in statistically significant increases in tumor uptake of embolic agents, and with significantly decreased uptake in normal liver tissue. The results were presented today at the Society of Interventional Radiology (SIR) meeting in Atlanta by investigator Austin Bourgeois, MD.
“While our research group was skeptical at the start of the study, the results were quite convincing. The Surefire catheter does indeed alter downstream distribution of microspheres in a potentially beneficial way compared to use of an end hole microcatheter,” said PI Alexander Pasciak, PhD, of the University of Tennessee Graduate School of Medicine. “Reviewing patient images showed a clear difference. The Surefire catheter increases microsphere deposition in the tumor, while decreasing uptake in normal liver tissue”
Nine patients received two embolizations on the same day and at the same infusion location with both the Surefire Infusion System and an end hole microcatheter. Half were treated first with the Surefire, the other half first with the end hole microcatheter. Nuclear imaging following each infusion allowed researchers to visualize the effect of different catheters on the distribution of hepatic emboli.
Protocol Assures Statistical Significance
“Having nine patients doesn’t seem like a lot, but the results are statistically significant because of the unique protocol employed where each patient acted as his or her own control,” Pasciak said. “The only variable was the catheter used.”
The protocol was adapted from same-day routine cardiac and renal perfusion studies.
“Patients in the study had widely varying conditions representing multiple types of liver cancer, and every stage,” Pasciak said. “We see zero downside and only potential upside to using Surefire. All patients had more uniform tumor coverage, increased dose deposition in tumor and decreased deposition in healthy liver tissue.”
Decreases in hepatic non-target embolization ranged from 24 to 89 percent, with a mean of 42 percent. Increased tumor deposition ranged from 33 to 90 percent, with a mean of 68 percent.
The authors hypothesize that with the anti-reflux Surefire catheter, a reduction in downstream pressure preferentially shunts particles toward the tumor compartment, temporarily increasing the tumor-to-normal uptake ratio.
The Surefire Infusion System is a first-in-class medical device with a unique expandable tip designed to minimize reflux and maximize direct-to-tumor delivery of cancer-fighting agents. It is used for diagnostic and therapeutic radioembolization or chemoembolization procedures.
About Surefire Medical
Surefire Medical, Inc., based in Westminster, Colo., was founded in 2009 to develop innovative infusion systems for the interventional radiology and interventional oncology markets. Surefire’s infusion systems are designed to precisely deliver embolic agents through a unique microcatheter with an expandable tip that collapses in forward flow and dynamically expands to the vessel wall in reverse flow in order to maximize targeted delivery, minimize reflux and reduce damage to healthy tissue. The Surefire Infusion System and specialty catheters have received regulatory approval in the U.S., Europe, Canada, Australia, New Zealand and Taiwan. www.surefiremedical.com